Expertise to develop even the most complex study designs

- Adaptive designs, seamless trials
- Basket and umbrella trials
- Historical data borrowing, synthetic control arm
- Biomarker-based designs
- Master protocols and platform trials
Machine-learning and artificial intelligence
- Biomarker discovery
- Semantic analyses
- Image analyses
Unique skillset in decision-making
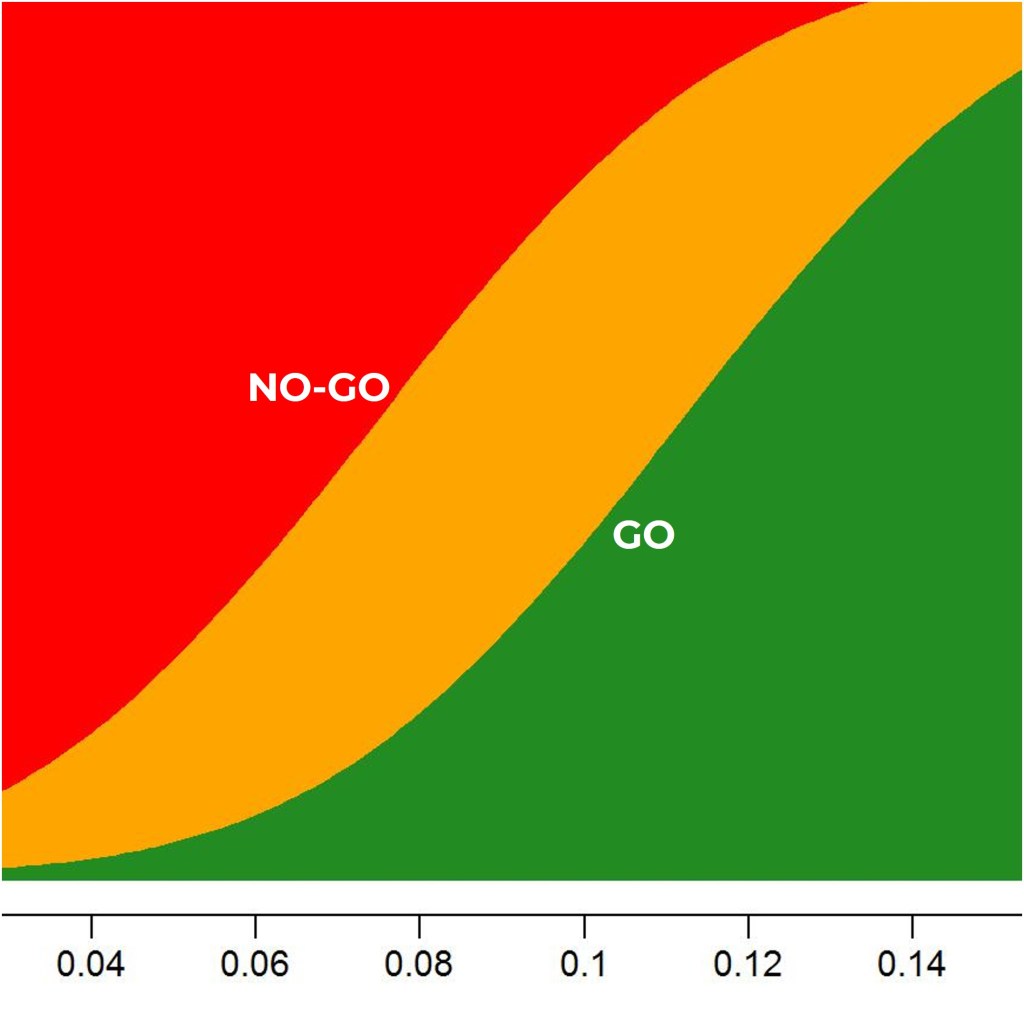
- Decision-making frameworks
- Probabilities of success
- Benefit-risk assessment
- Patient and expert elicitation
- Portfolio value profile
- Meta-analyses, evidence synthesis
Advanced statistical models
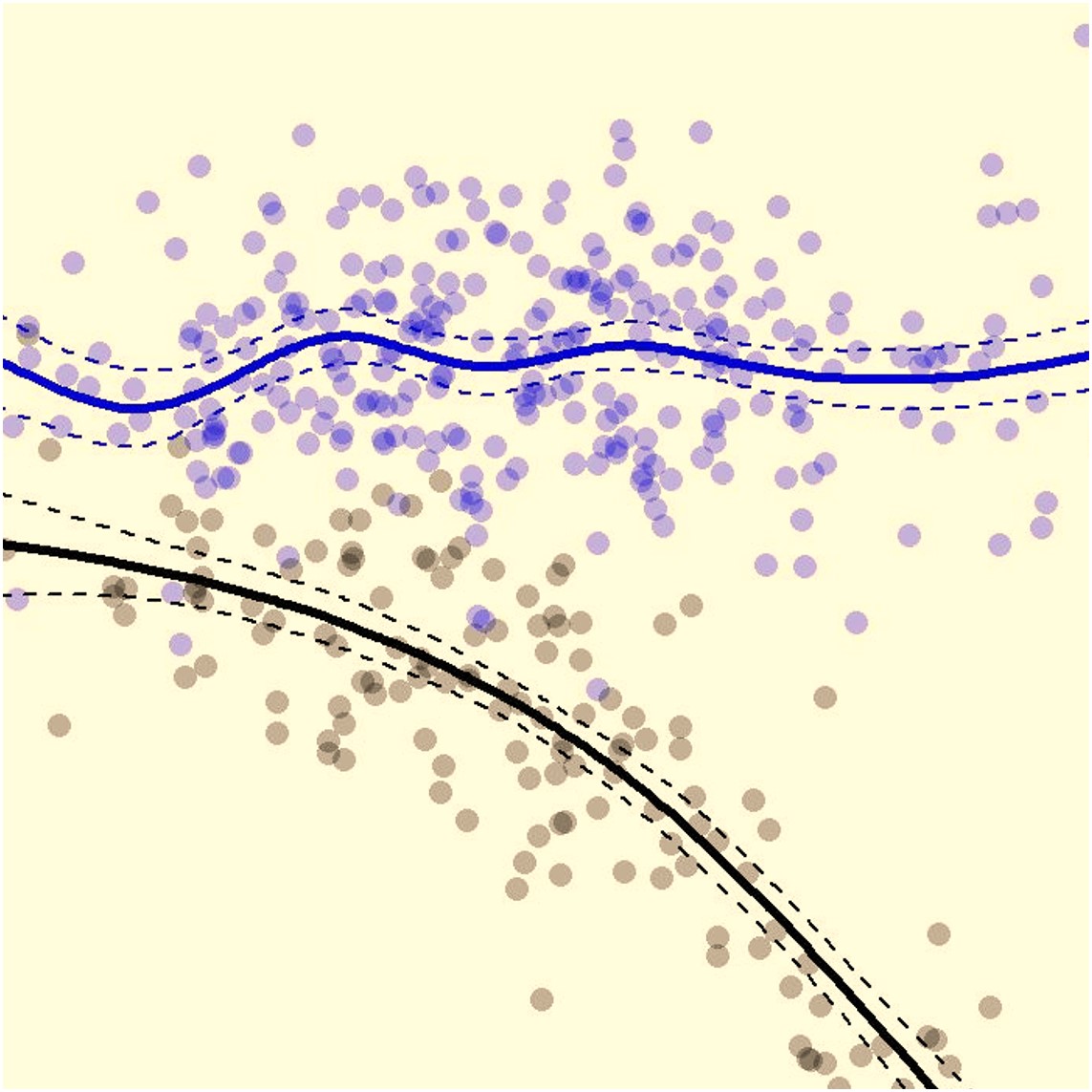
- Dose-response relationship
- Endpoint’s surrogacy
- Missing data
- Biomarkers
Superior knowledge of early phase dose-finding and dose-escalation trials
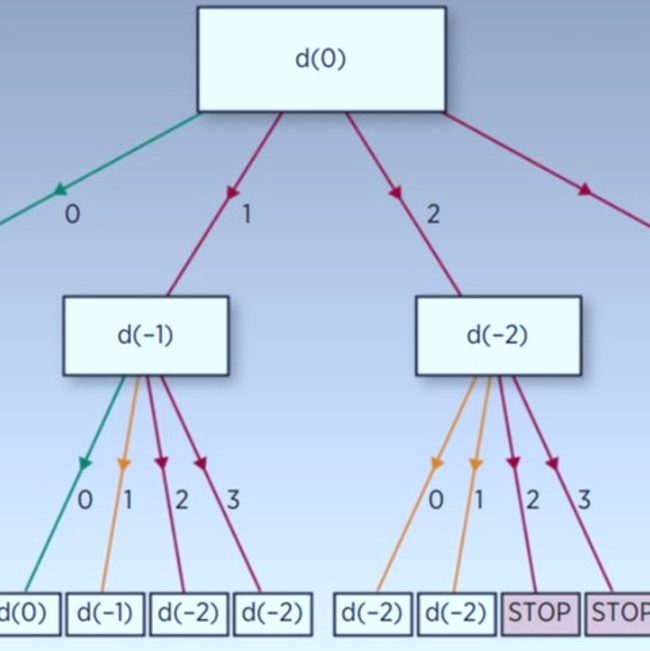
- Bayesian designs
- Combination trials
- Multiple objectives
Training courses and seminars for statisticians and clinicians

- Training courses for statisticians, with practicals in R
- Statistics for non-statisticians
- Participation in advisory boards and Data Monitoring Committees
- Independent statisticians for interim analyses of clinical trials
- Statistical support for regulatory applications (briefing books, regulatory meetings)
- Risk assessment for due-diligences
- To simplify the implementation of complex statistical methods
- To facilitate discussions with the project teams
Independent experts with an unbiased perspective on study designs and results

Development of
RShiny Apps


